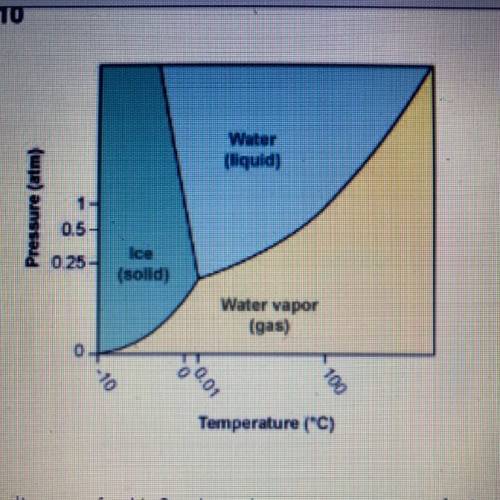
Using the phase diagram for H20, what phase is water in at 1 atm pressure
and 150°C?
A....


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Questions in other subjects:

History, 04.08.2019 20:30


English, 04.08.2019 20:30

History, 04.08.2019 20:30

History, 04.08.2019 20:30

History, 04.08.2019 20:30

Biology, 04.08.2019 20:30

Social Studies, 04.08.2019 20:30

Mathematics, 04.08.2019 20:30