
HELP ASAPPP
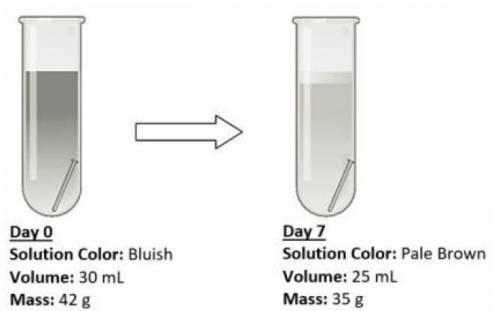
An iron nail was placed in an open test tube containing some salt solution, and then left for a week for observations. The experimental setup and observations are shown below.
Which of the following best demonstrates that a chemical change has taken place?
The salt solution was discolored after a week.
The volume of the salt solution has decreased.
The mass of the salt solution, beaker and nails decreased.
The iron nail is still undissolved in the solution after a week.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 23:30, hcllxxhhlpcj
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 03:30, elijahjacksonrp6z2o7
In general metals get as you move from left to right across the periodic table.
Answers: 1
You know the right answer?
HELP ASAPPP
An iron nail was placed in an open test tube containing some salt solution, and then le...
Questions in other subjects:

Mathematics, 20.10.2019 07:00



Social Studies, 20.10.2019 07:00


History, 20.10.2019 07:00


Mathematics, 20.10.2019 07:00


Mathematics, 20.10.2019 07:00



