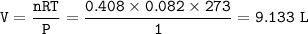Chemistry, 13.01.2021 07:10 tawna6988owtjg6
Trimethylamine, (CH3)2N is a weak base (K6 = 6.3 x 10-5). What volume of this gas, measured at STP, must be dissolved in 2.5 L of solution to give that solution a pOH of 2.50? PLEASE HELP ITS DUE IN 20 mins :

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, advancedgamin8458
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 06:30, AleciaCassidy
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

You know the right answer?
Trimethylamine, (CH3)2N is a weak base (K6 = 6.3 x 10-5). What volume of this gas, measured at STP,...
Questions in other subjects:




History, 14.07.2020 16:01

Mathematics, 14.07.2020 16:01



English, 14.07.2020 16:01


Mathematics, 14.07.2020 16:01

![\tt [OH^-]=10^{-2.5}=0.0032=3.2\times 10^{-3}](/tpl/images/1031/5783/b6571.png)
![\tt [OH^-]=\sqrt{Kb.M}\\\\(3.2\times 10^{-3})^2=6.3\times 10^{-5}\times M\\\\M=0.163](/tpl/images/1031/5783/8f3e1.png)