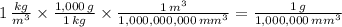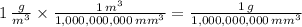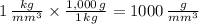Chemistry, 12.01.2021 18:30 gonzalesalexiaouv1bg
The same physical quantity, such as density, can be reported using different units. You found that water has a density of 1000kg/m^3=1g/cm^3 . Because the density of water must be the same regardless of what units you use to measure it, you can conclude that an object whose density is 1 kg/m^3 must be less dense than water. In other words, 1 kg/m^3 is less than 1 g/cm^3. If you had three different objects with densities of 1 kg/m^3 , 1 g/m^3 , and 1 kg/mm^3 , which object would be the most dense?

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:30, darkshaders11
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 01:00, tjeffers90028
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
The same physical quantity, such as density, can be reported using different units. You found that w...
Questions in other subjects:


Mathematics, 20.09.2020 17:01


Mathematics, 20.09.2020 17:01





Chemistry, 20.09.2020 17:01

Chemistry, 20.09.2020 17:01