
Chemistry, 12.01.2021 17:50 alexandrarosete7
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant R. Suppose the osmotic pressure of a certain solution is measured to be 22 atm at an absolute temperature of 353K. Write an equation that will let you calculate the molarity c of this solution. Your equation should contain only symbols. Be sure you define each symbol other than R.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:20, datboyjulio21
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 13:00, naomicervero
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute tempe...
Questions in other subjects:




English, 03.06.2021 17:10






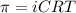
 = osmotic pressure exerted by a solution
= osmotic pressure exerted by a solution

