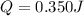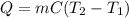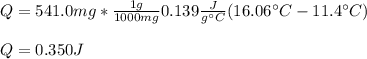Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 23:00, hailey5campbelp7d1c0
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 02:10, sativataurus
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2
You know the right answer?
Calculate the energy required to heat 541.0mg of mercury from 11.4°C to 16.6°C. Assume the specifi...
Questions in other subjects:

History, 16.10.2019 02:00

Mathematics, 16.10.2019 02:00

Physics, 16.10.2019 02:00


Biology, 16.10.2019 02:00


Spanish, 16.10.2019 02:00



Mathematics, 16.10.2019 02:00