Calculate the morality for the following
10.5 kg of Na2 SO4. 10H2O in 18.60 L...

Chemistry, 12.01.2021 16:50 zafarm2oxgpmx
Calculate the morality for the following
10.5 kg of Na2 SO4. 10H2O in 18.60 L

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2


Chemistry, 23.06.2019 02:30, micahwilkerson9495
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
Questions in other subjects:


Physics, 10.05.2021 08:30


Computers and Technology, 10.05.2021 08:30

Mathematics, 10.05.2021 08:30

Mathematics, 10.05.2021 08:30



Mathematics, 10.05.2021 08:30

Mathematics, 10.05.2021 08:30

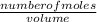
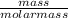
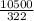 = 32.6mole
= 32.6mole 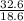 = 1.75M
= 1.75M

