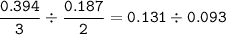Chemistry, 12.01.2021 14:00 24wilsleaann
If you react 25.0g of Cu with 25.0g of AlCl3 in the following reaction 3Cy + 2AlCl3 -> 3CuCl2 + 2Al
a. find the excess and limiting reactants
b. calculate the mass of leftover reactant

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, aedmund1225
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 10:30, mv603177
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

You know the right answer?
If you react 25.0g of Cu with 25.0g of AlCl3 in the following reaction 3Cy + 2AlCl3 -> 3CuCl2 + 2...
Questions in other subjects:

Social Studies, 25.11.2021 14:00

French, 25.11.2021 14:00


History, 25.11.2021 14:00




Mathematics, 25.11.2021 14:00

Mathematics, 25.11.2021 14:00