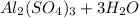Chemistry, 11.01.2021 22:30 JessTaylr04
For the following reaction, 3.04 grams of sulfuric acid are mixed with excess aluminum oxide. The reaction yields 2.53 grams of aluminum sulfate. aluminum oxide (s) sulfuric acid (aq) aluminum sulfate (aq) water (l) What is the theoretical yield of aluminum sulfate

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Priskittles
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 13:30, jcastronakaya
The zinc within a copper-plated penny dissolves in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can reach the zinc). the reaction between the acid and the zinc 2h+(aq)+zn(s)→h2(g)+zn2+(aq) . when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 °c is 0.947 l at a total pressure of 743 mmhg . (vapor pressure of water is 23.78 mmhg at 25 °c .) what mass of hydrogen gas is collected? answer in appropriate significant figures
Answers: 3

Chemistry, 23.06.2019 15:20, brandon0227
Which element below could be an isotope of berylliumsodium-10beryllium-10boron -9carbon-9
Answers: 2
You know the right answer?
For the following reaction, 3.04 grams of sulfuric acid are mixed with excess aluminum oxide. The re...
Questions in other subjects:

Business, 01.04.2021 21:30


English, 01.04.2021 21:30

Mathematics, 01.04.2021 21:30

Mathematics, 01.04.2021 21:30



Geography, 01.04.2021 21:30


English, 01.04.2021 21:30

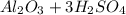 →
→