
Chemistry, 11.01.2021 14:00 okitsfrizz6366
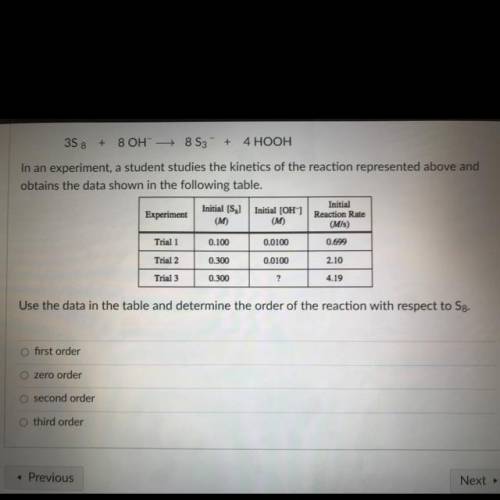
3S8 + 8 OH- + 8 S3 + 4 HOOH
In an experiment, a student studies the kinetics of the reaction represented above and
obtains the data shown in the following table.
Initial [S
Initial
Initial (OH)
Experiment
Reaction Rate
(M)
(M)
(M/s)
Trial 1
0.100
0.0100
0.699
Trial 2
0.300
0.0100
2.10
Trial 3
0.300
?
4.19
Use the data in the table and determine the order of the reaction with respect to Sg.
O first order
O zero order
O second order
O third order

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2


Chemistry, 22.06.2019 21:30, rileydavidharless
Which substance can be broken down by chemical means
Answers: 1
You know the right answer?
3S8 + 8 OH- + 8 S3 + 4 HOOH
In an experiment, a student studies the kinetics of the reaction repres...
Questions in other subjects:



Mathematics, 27.08.2019 03:30


Health, 27.08.2019 03:30

Geography, 27.08.2019 03:30

Mathematics, 27.08.2019 03:30

Mathematics, 27.08.2019 03:30

English, 27.08.2019 03:30

Spanish, 27.08.2019 03:30



