
Chemistry, 11.01.2021 04:10 makennahudson94
The boiling point of water is 100.0°C at 1 atmosphere.
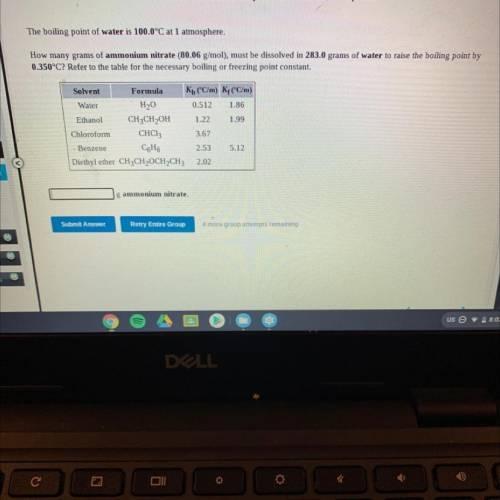
How many grams of ammonium nitrate (80.06 g/mol), must be dissolved in 283.0 grams of water to raise the boiling point by
0.350°C? Refer to the table for the necessary boiling or freezing point constant.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2


Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1

You know the right answer?
The boiling point of water is 100.0°C at 1 atmosphere.
How many grams of ammonium nitrate (80.06 g/...
Questions in other subjects:


Mathematics, 31.10.2019 04:31

Mathematics, 31.10.2019 04:31


English, 31.10.2019 04:31

Mathematics, 31.10.2019 04:31



Mathematics, 31.10.2019 04:31

Mathematics, 31.10.2019 04:31



