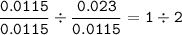Chemistry, 10.01.2021 20:20 Wolfgirl2032
Substance X is a compound containing 632mg of manganese and 368mg of oxygen. Substance X is shown
below. Manganese has a relative atomic mass of 55 and oxygen has a relative atomic mass of 16. What is the
value of y in the formula below?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 21:30, Lindsay882
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 23.06.2019 01:00, kwarwick0915
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 09:00, littlemoneyh
20 grams of water. she poured out 15 grams. which of the following physical properties of the water changes? a .boiling point b. density c .electrical conductivity d .volume
Answers: 2
You know the right answer?
Substance X is a compound containing 632mg of manganese and 368mg of oxygen. Substance X is shown
b...
Questions in other subjects:

Biology, 20.11.2020 18:40





Mathematics, 20.11.2020 18:40

Social Studies, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40