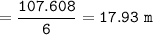Chemistry, 10.01.2021 16:40 emmadivaburnsox7ae9
Winter is coming and it's time to make sure you have the right amount of antifreeze
in your car. The best mixture of antifreeze and water is 50% antifreeze with 50%
water. The cooling system in your car has a mixture of 6.00 L water with 6.00 L of
ethylene glycol (antifreeze).
What is the molality of that solution?
The chemical formula for ethylene glycol is C2H602 and its density is 1.1132 g/cm3. (
Hint- use the density to find the mass of 6.0 L of C2H6O2, and remember that 1.0cm3
= 1.0 mL and that 1.0 L of water = 1.0 kg)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, iloveballet1857
The electron configuration for chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 instead of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 4 4 s 1 . the configuration is an exception to the
Answers: 3

Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1

Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
You know the right answer?
Winter is coming and it's time to make sure you have the right amount of antifreeze
in your car. Th...
Questions in other subjects:



History, 16.01.2021 19:30


Arts, 16.01.2021 19:30

Mathematics, 16.01.2021 19:30

Mathematics, 16.01.2021 19:30

Chemistry, 16.01.2021 19:30

Computers and Technology, 16.01.2021 19:30

Mathematics, 16.01.2021 19:30