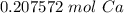How many moles of Ca are in 1.25x1023 atoms?
We used two conversion factors in this unit, they are provided below. It is your responsibility to know which conversion factor to use!
Avogadro’s number: 6.02x1023 atoms = 1 mole
Molar mass of calcium: 40.078 g Ca = 1 mol Ca
A. 3.12x10^21 mol
B. 0.21 mol
C. 3.12 mol
D. 0.21x10^21 mol

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 23.06.2019 03:50, mobslayer88
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
How many moles of Ca are in 1.25x1023 atoms?
We used two conversion factors in this unit, they are...
Questions in other subjects:

Mathematics, 27.01.2021 06:20

Mathematics, 27.01.2021 06:20


Mathematics, 27.01.2021 06:20

English, 27.01.2021 06:20


Mathematics, 27.01.2021 06:20

SAT, 27.01.2021 06:20

Mathematics, 27.01.2021 06:20

German, 27.01.2021 06:20

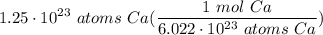 Multiply:
Multiply: