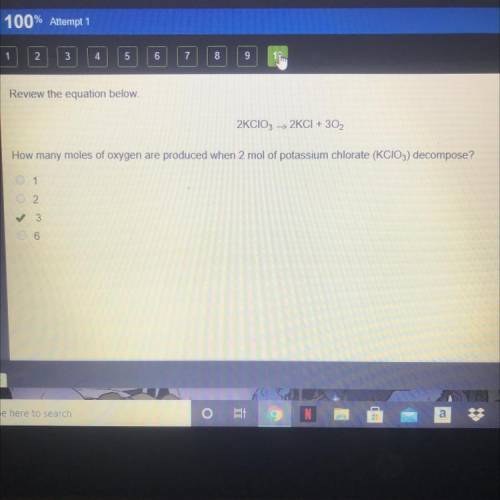
Review the equation below.
2KCIO3 > 2KCI + 3O2
How many moles of oxygen are produced...

Chemistry, 09.01.2021 22:10 tessalopezgarcia2345
Review the equation below.
2KCIO3 > 2KCI + 3O2
How many moles of oxygen are produced when 2 mol of potassium chlorate (KCIO3) decompose?
A. 1
B. 2
C. 3
D. 6
The correct answer is C on edge 2021

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
Questions in other subjects:


History, 13.04.2020 05:27


Mathematics, 13.04.2020 05:27


Medicine, 13.04.2020 05:29


Mathematics, 13.04.2020 05:30

Mathematics, 13.04.2020 05:30




