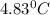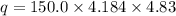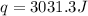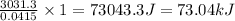2
3 of 6 - SCH4U
Date: Name(s):
4. HCl is a corrosive colourless gas that dissolves rea...

Chemistry, 09.01.2021 06:20 saleenhernandez83
2
3 of 6 - SCH4U
Date: Name(s):
4. HCl is a corrosive colourless gas that dissolves readily in water.
Aqueous HCl reacts with NaOH to form water and NaCl. In a simple
calorimeter, a 100.00 mL sample of 0.415 mol/L HCl(aq) is mixed
with 50.00 mL of excess NaOH(aq). During the reaction, there is a
rise in temperature by 4.83 °C. Calculate the molar enthalpy change
(in kJ/mol) for the above reaction. SHOW ALL YOUR WORK.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, sillslola816oxb5h7
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1


Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1
You know the right answer?
Questions in other subjects:



History, 20.10.2019 06:10



English, 20.10.2019 06:10

Social Studies, 20.10.2019 06:10

Mathematics, 20.10.2019 06:10

Chemistry, 20.10.2019 06:10

Mathematics, 20.10.2019 06:10


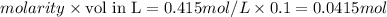
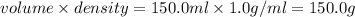
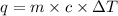
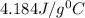
 = change in temperature =
= change in temperature =