Chemistry, 08.01.2021 21:40 jeffreyaxtell4039
Part D
In parts b and c, you measured the average mass of each group of pennies. Now you will measure their volume. ( for this part, assume that the pennies dated before and after 1982 have the same volume) on measuring volume before continuing
1. Add water to the narrow transparent container until it is about half full
2. Stick a piece of tape to the outside of the container to mark the water level. The water level must be even with the top edge of the tape.
3. Using a graduated cyclinder or a teaspoon measure, add another 5 ML (1 teaspoon) of water to the container. Be sure your measurement is exact
4. Stick another piece of tape to the outside of the container to mark the new water level. This time, the water level must be even with the bottom edge of the tape.
5. Next remove 5 mL (1 teaspoon) of water from the container. The water level will again be even with the top edge of the bottom piece of tape.
6. Add pennies one at a time until the water level is even with the lower edge of the top piece of tape. In the table record the number of pennies added . The pennies you added just displaced about 5 mL of water
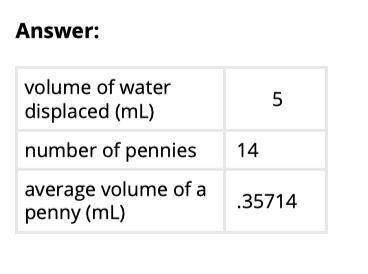
7. Divide 5 mL by the number of pennies you added to determine the average volume of each penny. Record this value on the table
Table
-volume of water displaced (mL) :
- number of pennies:
-average volume of a penny(mL) :

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, alaf05160
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks. energy was destroyed inside the blocks. energy was absorbed into the blocks from outside the system. energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 22.06.2019 22:30, jaylenmiller437
The diagram shows the relationship between scientific disciplines. the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a. physics b. biology c. chemistry d. metallurgy
Answers: 2
You know the right answer?
Part D
In parts b and c, you measured the average mass of each group of pennies. Now you will measu...
Questions in other subjects:

Mathematics, 24.06.2019 05:30


Chemistry, 24.06.2019 05:30

Chemistry, 24.06.2019 05:30

Chemistry, 24.06.2019 05:30


Mathematics, 24.06.2019 05:30


Chemistry, 24.06.2019 05:30

Mathematics, 24.06.2019 05:30