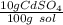Chemistry, 08.01.2021 17:10 danteyoungblood7
What is the molarity of a solution of 10% by mass cadmium sulfate, CdSO4 (molar mass = 208.46 g/mol) by mass? The density of the solution is 1.10 g/mL.
a) 0.528 M
b) 0.436 M
c) 0.479 M
d) 0.048 M
e) 22.9 M

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:50, sgslayerkingminecraf
Which of the following statements about acidic water is true? a. acid has no effect on the h, o molecules. b. the solution contains a larger number of oh ions than h, o ions. c. the solution contains a larger number of h, o ions than qh ions. d. the solution contains an equal number of h, o ions and oh ions. none of the above e.
Answers: 1


Chemistry, 23.06.2019 06:30, fshane7705
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
What is the molarity of a solution of 10% by mass cadmium sulfate, CdSO4 (molar mass = 208.46 g/mol)...
Questions in other subjects:

Mathematics, 10.02.2021 23:40

Mathematics, 10.02.2021 23:40

Arts, 10.02.2021 23:40



Mathematics, 10.02.2021 23:40

English, 10.02.2021 23:40

Biology, 10.02.2021 23:40