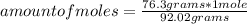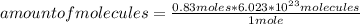Chemistry, 07.01.2021 18:30 orangeicecream
How many molecules of N2O4 are in 76.3g N2O4? The molar mass of N2O4 is 92.02 g/mol.
a. 4.59 × 10^25 N2O4 molecules
b. 5.54 × 10^25 N2O4 molecules
c. 7.26 × 10^23 N2O4 molecules
d. 1.38 × 10^24 N2O4 molecules
e. 4.99 × 10^23 N2O4 molecules

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1


Chemistry, 23.06.2019 04:10, angelina6836
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2

Chemistry, 23.06.2019 07:10, skatingflower
What additive are in a lavender tube for phlebotomists
Answers: 1
You know the right answer?
How many molecules of N2O4 are in 76.3g N2O4? The molar mass of N2O4 is 92.02 g/mol.
a. 4.59 × 10^2...
Questions in other subjects:


Physics, 13.09.2019 07:10

English, 13.09.2019 07:10


History, 13.09.2019 07:10

Mathematics, 13.09.2019 07:10



Advanced Placement (AP), 13.09.2019 07:10

History, 13.09.2019 07:10