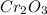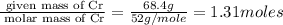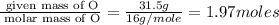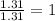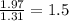Chemistry, 07.01.2021 17:30 elizabethatkins1922
Measurements show that unknown compound X has the following composition: element mass % chromium 68.4% oxygen 31.5% Write the empirical chemical formula of X.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, kiaramccurty
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 23.06.2019 00:00, savyblue1724707
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
You know the right answer?
Measurements show that unknown compound X has the following composition: element mass % chromium 68....
Questions in other subjects:




History, 04.04.2020 05:30





Biology, 04.04.2020 05:30