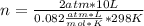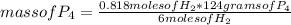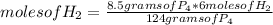The moles of PH₃ produced are 0.2742 and the total number of moles of gas present at the end of the reaction is 0.6809.
Explanation:
Phosphorus reacts with H₂ according to the balanced equation:
P₄ (s) + 6 H₂ (g) ⇒ 4 PH₃ (g)
By stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of each compound participate in the reaction:
P₄: 1 moleH₂: 6 molesPH₃:4 moles
Being the molar mass of the compounds:
P₄: 124 g/moleH₂: 2 g/molePH₃: 34 g/mole
The following mass amounts of each compound participate in the reaction:
P₄: 1 mole* 124 g/mole= 124 gH₂: 6 mole* 2 g/mole= 12 gPH₃: 4 moles* 34 g/mole= 136 g
An ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them constitutes the ideal gas law, an equation that relates the three variables if the amount of substance, number of moles n, remains constant and where R is the molar constant of the gases:
P * V = n * R * T
In this case you know:
P= 2 atmV= 10 Ln= ?R= 0.082
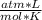
T= 298 K
Replacing:
2 atm*10 L= n*0.082 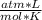 *298 K
*298 K
and solving you get:
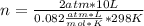
n=0.818 moles
The limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
To determine the limiting reagent, you can use a simple rule of three as follows: if 6 moles of H₂ react with 124 g of P₄, 0.818 moles of H₂ with how much mass of P₄ will it react?
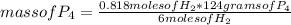
mass of P₄= 16.90 grams
But 16.90 grams of P₄ are not available, 8.50 grams are available. Since you have less mass than you need to react with 0.818 moles of H₂, phosphorus P₄ will be the limiting reagent.
Then you can apply the following rules of three:
If 124 grams of P₄ produce 4 moles of PH₃, 8.50 grams of P₄, how many moles do they produce?

moles of PH₃=0.2742
If 124 grams of P₄ react with 6 moles of H₂, 8.50 grams of P₄ with how many moles of H₂ do they react?
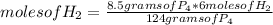
moles of H₂= 0.4113
If you have 0.818 moles of H₂, the number of moles of gas H₂ present at the end of the reaction is calculated as:
0.818 - 0.4113= 0.4067
Then the total number of moles of gas present at the end of the reaction will be the sum of the moles of PH₃ gas and H₂ gas that did not react:
0.2742 + 0.4067= 0.6809
Finally, the moles of PH₃ produced are 0.2742 and the total number of moles of gas present at the end of the reaction is 0.6809.



















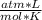 T= 298 K
T= 298 K