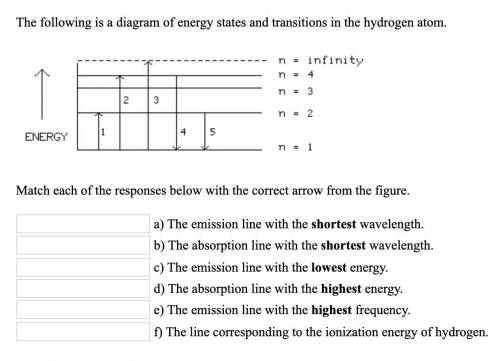
Chemistry, 07.01.2021 17:10 ScarySkyler5571
g The emission line with the longest wavelength. 2.) The absorption line with the shortest wavelength. 3.) The emission line with the highest energy. b 4.) The absorption line with the highest energy. a 5.) The emission line with the lowest frequency. a 6.) The line corresponding to the ionization energy of hydrogen.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 15:00, asims13
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 04:00, dustinsampsin2486
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3
You know the right answer?
g The emission line with the longest wavelength. 2.) The absorption line with the shortest wavelengt...
Questions in other subjects:

Mathematics, 22.07.2019 10:00

History, 22.07.2019 10:00

History, 22.07.2019 10:00




Computers and Technology, 22.07.2019 10:00



Mathematics, 22.07.2019 10:00