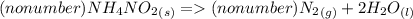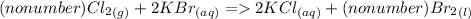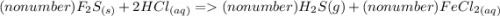ANSWER ASAP
1. NH₄NO₂(s) → N₂(g) + H₂O(l)
1a. Write the number (if any) that should go i...

ANSWER ASAP
1. NH₄NO₂(s) → N₂(g) + H₂O(l)
1a. Write the number (if any) that should go in front of NH₄NO₂(s), N₂(g), and H₂O(l) in order with commas in between. If there shouldn't be a number, write "no number".
1b. What type of reaction is represented in this equation?
2. Cl₂(g) + KBr(aq) → KCl (aq) + Br₂(l)
2a. Write the number (if any) that should go in front of each compound in order with commas in between. If there shouldn't be a number, write "no number".
2b. What type of reaction is represented in this equation?
3. FeS(s) + HCl(aq) → H₂S(g) + FeCl₂ (aq)
3a. Write the number (if any) that should go in front of each compound in order with commas in between. If there shouldn't be a number, write "no number".
3b. What type of reaction is represented in this equation?

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 12:30, okasiafolk27
15) a substance used in manufacturing gasoline consists of finely divided platinum supported on an inert solid. suppose that the platinum is formed by the high temperature reaction between platinum (iv) oxide and hydrogen gas. the other product is water. a) write and balance the equation b) how many grams of hydrogen are needed to produce 1.0 g of platinum metal? c) how many moles of water are produced at the same time? how many grams? ( show work, .)
Answers: 1
You know the right answer?
Questions in other subjects:

Social Studies, 31.08.2019 06:10



History, 31.08.2019 06:10

World Languages, 31.08.2019 06:10




History, 31.08.2019 06:10