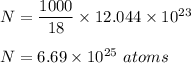Chemistry, 07.01.2021 05:20 walkerobrien5
18.0 mL of water contains 6.022 x 1023 water molecules. How many hydrogen atoms are in 1.00 L of water? (Each water molecule, H2O, contains two hydrogen atoms.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alaina3792
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2


Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1
You know the right answer?
18.0 mL of water contains 6.022 x 1023 water molecules. How many hydrogen atoms are in 1.00 L of wat...
Questions in other subjects:


Mathematics, 12.03.2021 23:00

Mathematics, 12.03.2021 23:00

History, 12.03.2021 23:00

English, 12.03.2021 23:00

Mathematics, 12.03.2021 23:00

Mathematics, 12.03.2021 23:00

History, 12.03.2021 23:00

Mathematics, 12.03.2021 23:00

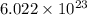 water molecules.
water molecules.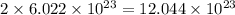 .
.