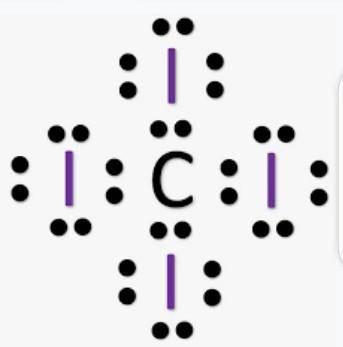
Chemistry, 07.01.2021 02:10 ricksterv5000
How do you draw the molecule Cl4 with its Lewis Structure?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:20, datboyjulio21
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2



Chemistry, 23.06.2019 01:00, breemills9552
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1
You know the right answer?
How do you draw the molecule Cl4 with its Lewis Structure?...
Questions in other subjects:


Mathematics, 31.05.2021 22:50



English, 31.05.2021 22:50




English, 31.05.2021 22:50