BRAINLIEST - ANSWER QUICKLY
Question 1 (1 point)
Saved
Which of the followin...

Chemistry, 06.01.2021 16:10 michellemunoz250
BRAINLIEST - ANSWER QUICKLY
Question 1 (1 point)
Saved
Which of the following is the BEST evidence of a chemical change?
Question 1 options:
Two solids forming a mixture.
Two liquids forming layers instead of mixing.
Two liquids mixing to produce a precipitate.
Two liquids mixing to blend their colors.
Question 2 (1 point)
Saved
Which of the following is NOT an example of a physical change?
Question 2 options:
A metal rod bends.
A metal rod is cut into two metal rods.
A metal rod dissolves in acid.
A metal rod is stretched longer.
Question 3 (1 point)
Saved
In a cup of liquid water, when would the water molecules stop moving?
Question 3 options:
The molecules would stop moving if the liquid water in the cup became still.
The molecules would not stop moving in the cup of liquid water.
The molecules would stop moving if the liquid water in the cup became a solid.
The molecules would stop moving if the liquid water in the cup became a gas.
Question 4 (1 point)
Saved
In which state(s) of matter could a substance have thermal energy?
Question 4 options:
Only liquids and gases
Only liquids and solids
Only liquids
Liquids, solids, and gases
Question 5 (1 point)
Saved
Which of the following is NOT an example of a chemical change?
Question 5 options:
Baking soda and vinegar foam and erupt like a volcano.
Water evaporates into steam.
A hand warmer heats up after you squeeze it.
An antacid tablet neutralizes stomach acid.
Question 6 (2 points)
Saved
Question 6 options:
Boyle’s Law: When
temperature
is held constant, the pressure and volume of a gas are
inversely
proportional.
Question 7 (2 points)
Saved
Question 7 options:
Anytime that a substance changes size, shape, or temperature it would be considered a
physical
change. But, if it changed properties such as its density, pH, or how it reacts with other substances it is most likely a
chemical
change.
Question 8 (2 points)
Saved
Use the diagram below to correctly match the statements that follow.
Question 8 options:
1
Has the most kinetic energy
3
Has the least kinetic energy
1
Has an undefined volume and shape
3
Has a defined shape and volume
2
Has a defined volume but not a defined shape
1.
A
2.
B
3.
C
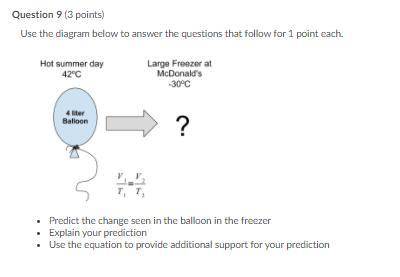
Question 9 (3 points)
Use the diagram below to answer the questions that follow for 1 point each.
Predict the change seen in the balloon in the freezer
Explain your prediction
Use the equation to provide additional support for your prediction
Question 9 options:
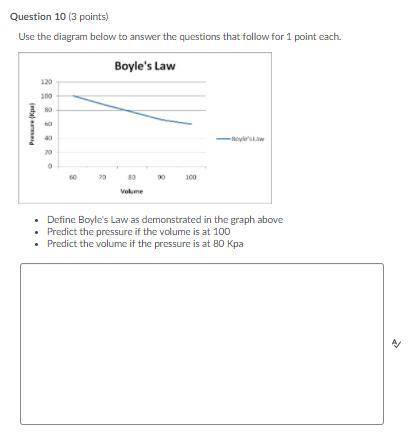
Question 10 (3 points)
Use the diagram below to answer the questions that follow for 1 point each.
Define Boyle's Law as demonstrated in the graph above
Predict the pressure if the volume is at 100
Predict the volume if the pressure is at 80 Kpa
Question 10 options:
Question 11 (3 points)
Use the diagram below to address the following questions for 1 point each:
Identify the beaker with the LOWEST amount of kinetic energy
Identify the beaker with the HIGHEST amount of kinetic energy
Explain the relationship between kinetic energy and thermal energy
Question 11 options:
Question 12 (6 points)
A balloon holds 50.0 mL of nitrogen at 25° C and a pressure of 736 mm Hg.
Answer the following questions for 2 points each:
According to Boyle's Law, what would have to change to double the pressure in the balloon?
According to Charles' Law, what would have to change to double the volume of the balloon?
Explain how the kinetic energy of molecules explains both Boyle's and Charles' Laws.



Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 05:00, xxaurorabluexx
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2

Chemistry, 23.06.2019 08:00, yddlex
Suppose a pair of chemical compounds a and b can react in two different ways: a + b -> c reaction 1 gives product c. a + b -> d reaction 2 gives product d. the following facts are known about the two reactions: . reaction 1 is endothermic and reaction 2 is exothermic. if a reaction vessel is charged (filled) with a and b , then at first d is produced faster than c. use these facts to sketch a qualitative reaction energy diagram for both reactions. note: because these sketches are only qualitative, the energies don? t have to be exact. they only have to have the right relationship to each other. for example, if one energy is less than another, that fact should be clear in your sketch.
Answers: 3
You know the right answer?
Questions in other subjects:



Mathematics, 20.04.2020 01:42





Mathematics, 20.04.2020 01:42

Health, 20.04.2020 01:42

Social Studies, 20.04.2020 01:42



