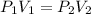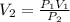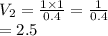Chemistry, 05.01.2021 20:20 MorallyGray
A balloon is filled with helium gas has a volume of 1.0 L at a pressure of 1.0 atm. The
balloon is released and reaches an altitude where the pressure is now 0.4 atm. What is the
new volume of the balloon at this altitude assuming the air temperature has not changed?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, johnnydenali67
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 03:00, bobbycisar1205
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1
You know the right answer?
A balloon is filled with helium gas has a volume of 1.0 L at a pressure of 1.0 atm. The
balloon is...
Questions in other subjects:

Business, 04.08.2019 08:30


Social Studies, 04.08.2019 08:30

History, 04.08.2019 08:30