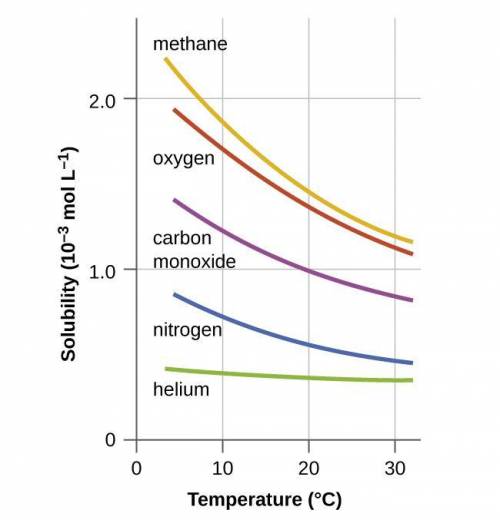
Chemistry, 05.01.2021 16:50 oneicyahdaley10
A solution is saturated in CO2 gas and KNO3 at room temperature. What happens when the solution is warmed to 75°C?
solid KNO, precipitates out of the solution
gaseous CO2 bubbles out of the solution
solid KNO, precipitates out and gaseous CO2 bubbles out
nothing happens; both CO2 and KNO3 remain in solution

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:10, mistiehaas
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2


Chemistry, 22.06.2019 09:30, psychocatgirl1
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone, due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3
You know the right answer?
A solution is saturated in CO2 gas and KNO3 at room temperature. What happens when the solution is w...
Questions in other subjects:

Mathematics, 22.03.2021 15:50


Mathematics, 22.03.2021 16:00