
Chemistry, 05.01.2021 16:40 Skylar4483
According to this balanced chemical equation,
what volume of C2H2 is required to form 40.0L
of CO2?
2C2H2(g) + 502(g) → 2H2O(g) + 4CO2(g)
A. 20.0L
B. 44.8L
C. 80.0L
D. 100 L

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, pennygillbert
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1



Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
According to this balanced chemical equation,
what volume of C2H2 is required to form 40.0L
Questions in other subjects:

Mathematics, 08.10.2019 09:30



Computers and Technology, 08.10.2019 09:30

English, 08.10.2019 09:30

Mathematics, 08.10.2019 09:30

History, 08.10.2019 09:30

Mathematics, 08.10.2019 09:30


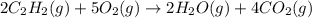
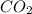 is formed by = 2 L of
is formed by = 2 L of 
 of
of 


