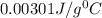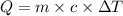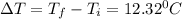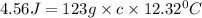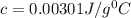Chemistry, 05.01.2021 16:30 tristanlindor5329
What is the specific heat of a 123 g substance that requires 4.56 J of heat in
order to increase its temperature by 12.32 °C?
A) 0.00301 J/g °C
B) 0.457 J/g °C
0 6910 J/g °C
D) 2.19 J/g °C
E) 0.0220 J/g°C

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 23.06.2019 05:00, kayranicole1
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1

Chemistry, 23.06.2019 05:00, shealynh52
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2

Chemistry, 23.06.2019 15:30, Marliii363782
An isotope undergoes radioactive decay. the new isotope that forms has an atomic number fhat is 2 less than the original isotopes. which kind of decay has occured and how do you know
Answers: 2
You know the right answer?
What is the specific heat of a 123 g substance that requires 4.56 J of heat in
order to increase it...
Questions in other subjects:


Computers and Technology, 10.05.2021 21:50


Chemistry, 10.05.2021 21:50



Mathematics, 10.05.2021 21:50

Mathematics, 10.05.2021 21:50

Mathematics, 10.05.2021 21:50

Mathematics, 10.05.2021 21:50