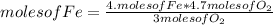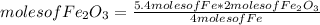Chemistry, 05.01.2021 16:30 gennhill14
If 5.4 moles of Fe react with 4.7 moles of O2, what is the maximum amount of Fe2O3 (in moles) that can be produced? What is the limiting reactant?
a
3.1 moles of Fe2O3 is the maximum amount that can be produced. Oxygen is the limiting reactant.
b
2.7 moles of Fe2O3 is the maximum amount that can be produced. Iron is the limiting reactant.
c
7.1 moles of Fe2O3 is the maximum amount that can be produced. Oxygen is the limiting reactant.
d
10.8 moles of Fe2O3 is the maximum amount that can be produced. Iron is the limiting reactant.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, cicimarie2018
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

You know the right answer?
If 5.4 moles of Fe react with 4.7 moles of O2, what is the maximum amount of Fe2O3 (in moles) that c...
Questions in other subjects:



Mathematics, 02.12.2020 01:40

Mathematics, 02.12.2020 01:40

Chemistry, 02.12.2020 01:40

History, 02.12.2020 01:40

Mathematics, 02.12.2020 01:40

Mathematics, 02.12.2020 01:40

Mathematics, 02.12.2020 01:40

Mathematics, 02.12.2020 01:40