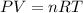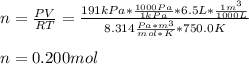Chemistry, 05.01.2021 16:20 parkerfreeze
If an instant pot has an internal pressure of 191.0 kPa, temperature of 750.0 K and a volume of 6.5 L, how many moles of gas would be contained inside the instant pot?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Arealbot
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 00:20, TamB01
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 03:00, litttyyyu33411
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2
You know the right answer?
If an instant pot has an internal pressure of 191.0 kPa, temperature of 750.0 K and a volume of 6.5...
Questions in other subjects:



Biology, 01.08.2019 02:30




Social Studies, 01.08.2019 02:30

English, 01.08.2019 02:30