
Consider the following equilibrium: 2SO^2(g) + O2(9) = 2 SO3^(g)
1. What is equal at equilibrium?
2. What would happen to the forward rate if some 0were removed from this equilibrium?
3. Explain why, in terms of collision theory.
4. Would the reaction still be at equilibrium at this point?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, Amandachavez94
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 22:00, Porciabeauty6788
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 22.06.2019 23:00, Kykebailey2356
Which of two curves exhibits exponential growth
Answers: 1
You know the right answer?
Consider the following equilibrium: 2SO^2(g) + O2(9) = 2 SO3^(g)
1. What is equal at equilibrium?
Questions in other subjects:

History, 04.01.2020 10:31

Mathematics, 04.01.2020 10:31




Mathematics, 04.01.2020 10:31




Biology, 04.01.2020 10:31

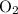 is removed from the mixture. The reason is that collisions between
is removed from the mixture. The reason is that collisions between 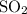 molecules and
molecules and 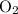 molecules would become less frequent.
molecules would become less frequent. 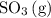 to the system, the backward reaction would have broken down the same amount of
to the system, the backward reaction would have broken down the same amount of 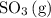 . So is the case for
. So is the case for 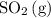 and
and 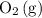 .
.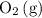 molecules would be present in the mixture, there would be fewer collisions (fruitful or not) between
molecules would be present in the mixture, there would be fewer collisions (fruitful or not) between 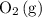 molecules in unit time. Even if the percentage of fruitful collisions stays the same, there would fewer fruitful collisions in unit time. It would thus appear that the forward reaction has become slower.
molecules in unit time. Even if the percentage of fruitful collisions stays the same, there would fewer fruitful collisions in unit time. It would thus appear that the forward reaction has become slower. 

