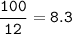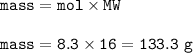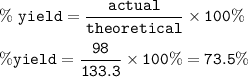Chemistry, 04.01.2021 05:50 Annabel9554
For the reaction C+2H 2 —->CH 4 calculate the percent yield if 98 g of methane is produced when 100. g of carbon reacts with an excess of hydrogen?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 19:30, youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2


Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
For the reaction C+2H 2 —->CH 4 calculate the percent yield if 98 g of methane is produced when 1...
Questions in other subjects:

Mathematics, 07.11.2020 21:50


History, 07.11.2020 21:50




Mathematics, 07.11.2020 21:50

Mathematics, 07.11.2020 21:50


English, 07.11.2020 21:50