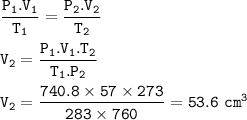Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, aubreykenzie686
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1


Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
A certain mass of hydrogen gas collected over water at 10°C and 750mmHg pressure has a volume of 57c...
Questions in other subjects:

Mathematics, 20.09.2020 08:01




Mathematics, 20.09.2020 08:01


Chemistry, 20.09.2020 08:01

History, 20.09.2020 08:01

Arts, 20.09.2020 08:01

Chemistry, 20.09.2020 08:01