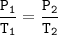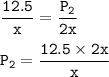Chemistry, 02.01.2021 20:10 kaylallangari1509
Without completing the calculations, determine what the new pressure will be in the problem below. Also,
explain how you were able to determine the new pressure without completing the calculations. Pay special
attention to how the temperature is changing in the problem.
A sample of ozone (0:2) is stored at x kelvin and 12.5 Pa. If the temperature is doubled to 2x kelvin,
what will the new
pressure be?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 12:00, luffybunny
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
You know the right answer?
Without completing the calculations, determine what the new pressure will be in the problem below. A...
Questions in other subjects:



Mathematics, 02.10.2019 03:00


History, 02.10.2019 03:00

Mathematics, 02.10.2019 03:00

History, 02.10.2019 03:00

Mathematics, 02.10.2019 03:00


English, 02.10.2019 03:00