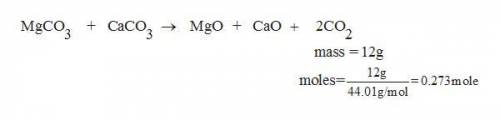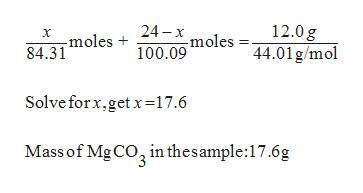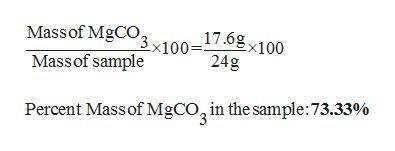30. A solid sample contains only CaCO3, and MgCO3.
To completely react the CaCO3 and MgCO3
pre...

Chemistry, 01.01.2021 20:00 joleiswan9919
30. A solid sample contains only CaCO3, and MgCO3.
To completely react the CaCO3 and MgCO3
present in the sample. 42.00 cm of 0.088 M
HCI were required. The anhydrous chloride salts
from the reaction, obtained by evaporation of
the filtrate weighed 0.19 g. The mass of CaCO3
present in the solid sample is (C = 12,0 = 16,
Mg = 24, Ca = 40, CI = 35.5 )
(1) 0.05 g.
(2) 0.07 g
(3) 0.09 g
(4) 0.11g
(5) 0.12 g

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 23.06.2019 03:00, makayyafreeman
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 16.12.2019 01:31


English, 16.12.2019 01:31

Mathematics, 16.12.2019 01:31

Mathematics, 16.12.2019 01:31



Geography, 16.12.2019 01:31