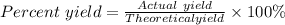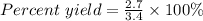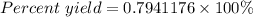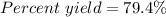Chemistry, 01.01.2021 04:50 timothycarter342
Sulfuric acid, H 2 S O 4 , is an important industrial chemical, typically synthesized in a multi-step process. What is the percent yield if a batch of H 2 SO 4 has a theoretical yield of 3.4 kg, and 2.7 kg are obtained at the end of the process

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3


Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Sulfuric acid, H 2 S O 4 , is an important industrial chemical, typically synthesized in a multi-ste...
Questions in other subjects:

World Languages, 23.10.2019 03:20

Spanish, 23.10.2019 03:20

Social Studies, 23.10.2019 03:20


Mathematics, 23.10.2019 03:20

Chemistry, 23.10.2019 03:20


English, 23.10.2019 03:20

English, 23.10.2019 03:20

History, 23.10.2019 03:20