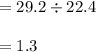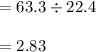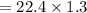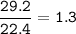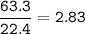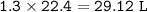Methane gas combusts according to the following
chemical equation:
CH4 (8) + 202(g) → CO2(g) + 2H2O(l)
29.2 L of methane gas is combusted with 63.3 L of
oxygen gas at STP. What volume of carbon dioxide
is produced in the reaction?
___L CO2
Your answer should be rounded to three significant figures. Do
not include units in your answer.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1


Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
Methane gas combusts according to the following
chemical equation:
CH4 (8) + 202(g) → CO2(g)...
CH4 (8) + 202(g) → CO2(g)...
Questions in other subjects:

Mathematics, 07.05.2020 02:04

Mathematics, 07.05.2020 02:04