
Chemistry, 31.12.2020 03:00 Division101
The Lewis diagram for BH2 is:
[H-B-H]-
The electron- pair geometry around the B atom in BH2 is:.
There arelone pair(s) around the central atom, so the geometry of BH2 is .

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1


Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 23.06.2019 01:20, michellectucker1982
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
The Lewis diagram for BH2 is:
[H-B-H]-
The electron- pair geometry around the B atom in BH2...
The electron- pair geometry around the B atom in BH2...
Questions in other subjects:

History, 23.06.2019 20:50



Mathematics, 23.06.2019 20:50



Chemistry, 23.06.2019 20:50


Mathematics, 23.06.2019 20:50

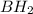 is called the trigonal planar. In this case, there is a pair of isolated electrons around Boron, which is the central atom, allowing the
is called the trigonal planar. In this case, there is a pair of isolated electrons around Boron, which is the central atom, allowing the 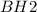 molecule to have a folded geometry.
molecule to have a folded geometry.


