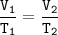temperature will change from

Chemistry, 31.12.2020 02:30 Elliendc7939
When the volume of a gas is
changed from 3.6 L to 15.5 L, the
temperature will change from
°C to 87°C.
Assume that the number of moles and the
pressure remain constant.
Be sure to notice that temperatures are
given in °C!


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, I1YBYY
Infants born with severe respiratory problems are sometimes given liquid ventilation: they breathe a liquid that can dissolve more oxygen than air can hold. one of these liquids is a fluorinated compound, cf3(cf2)7br. the solubility of oxygen in this liquid is 66 mlo2 per 100 ml liquid. in contrast, air is 21 % oxygen by volume. calculate the moles of o2 present in an infant's lungs (volume: 12 ml ) if the infant takes a full breath of air. assume a pressure of 1 atm in the lungs.
Answers: 1

Chemistry, 21.06.2019 16:30, TheRealKodakBlack
What is a scientific theory? a. a scientist's guess about how something works b. the results of an experiment obtained using the scientific method c. a proven fact that will never change d. an idea that is backed by data from many sources
Answers: 2


Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2
You know the right answer?
When the volume of a gas is
changed from 3.6 L to 15.5 L, the
temperature will change from
temperature will change from
Questions in other subjects:

Spanish, 14.04.2021 17:40




English, 14.04.2021 17:40

Health, 14.04.2021 17:40



Mathematics, 14.04.2021 17:40