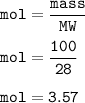Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1


Chemistry, 23.06.2019 03:30, HalpMahOnMahH0meW0rk
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
3.Hydrogen reacts with nitrogen to produce ammonia according to the equation:
3H2(g) + N2(g) 2NH3...
Questions in other subjects:



Chemistry, 29.01.2021 20:50

Mathematics, 29.01.2021 20:50






Mathematics, 29.01.2021 21:00