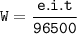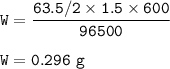Chemistry, 29.12.2020 15:10 nerdypineapple
6.A solution of CuSO4 is electrolyzed for 10 minutes with a current of 1.5 amperes. What is the mass of copper deposited at the cathode? ( F =96,500C, Charge of Cu in CuSO4 = +2)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

You know the right answer?
6.A solution of CuSO4 is electrolyzed for 10 minutes with a current of 1.5 amperes. What is the mass...
Questions in other subjects:

Mathematics, 18.12.2020 23:00






Advanced Placement (AP), 18.12.2020 23:00

Arts, 18.12.2020 23:00