
Chemistry, 28.12.2020 08:00 Aliciaonfleek
A gaseous mixture of 1.0 mol of 12 and 1.0 mol of H2 was placed in an empty vessel that has a
volume of 2.0 L. The system was allowed to reach dynamic equilibrium at 300°C.
a) State briefly what happened to the initial concentration of 12 and H2 as the reaction reaches
equilibrium
b) Does the reaction stoop at equilibrium? Why?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 10:00, 2019reynolds
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1
You know the right answer?
A gaseous mixture of 1.0 mol of 12 and 1.0 mol of H2 was placed in an empty vessel that has a
volum...
Questions in other subjects:

Computers and Technology, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50

Chemistry, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50

History, 17.03.2021 23:50




Health, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50

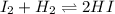
![\frac{[HI]^2}{[I_2][H_2]}](/tpl/images/1008/4126/2a174.png)


