

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 02:30, ulilliareinhart2
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2


Chemistry, 23.06.2019 05:00, MoneyMike42
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
1.72 moles of NOCI were placed in a 2.50 L reaction chamber
at 427°C. After equilibrium was reached...
Questions in other subjects:

Mathematics, 06.04.2020 00:47

Mathematics, 06.04.2020 00:47



Mathematics, 06.04.2020 00:47






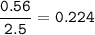

![\tt Kc=\dfrac{[NO]^2[Cl_2]}{[NOCl]^2}\\\\Kc=\dfrac{0.224^2\times 0.112}{0.464^2}=0.026](/tpl/images/1007/8914/ad1b4.png)


