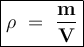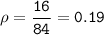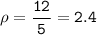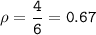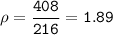Chemistry, 24.12.2020 01:00 QueenxBoujee
His table shows the mass and volume of four different objects.
A two-column table with 4 rows. The first column titled objects has entries W, X, Y, Z. The second column titled Measurements has entries Mass: 16 grams Volume: 84 centimeters cubed in the first cell, Mass: 12 grams Volume: 5 centimeters cubed in the second cell, Mass: 4 grams Volume: 6 centimeters cubed in the third cell, Mass: 408 grams Volume: 216 centimeters cubed in the fourth cell.
Which ranks the objects from most to least dense?
X, Y, W, Z
X, Z, Y, W
W, Y, Z, X
Z, Y, X, W

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, mrylenastewart
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 08:30, vanessadaniellet21
Since the gas in your graduated cylinder is a mixture of butane and water vapor, you must determine the partial pressure of the butane, pbutane, alone. to do this, consult a reference and record the partial pressure of the water vapor, pwater, at the temperature you recorded. use the following formula to compute the partial pressure of the butane. pbutane = atmosphere - pwater use the following combined gas law formula and compute the volume that the butane sample will occupy at stp. (hint: convert both temperatures to kelvin.) pbutane x voriginal = pstandard x vfinal troom tstandard use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. grams of butane you used “x” grams of butane ml of butane corrected to stp = 22,400 ml compute the theoretical molar mass of butane based on its formula and the atomic masses on the periodic table. compare your experimental results from #3 to the theoretical value of #4, computing a percent error of your findings using this formula: % error = measured value - accepted value x 100 accepted value use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. need asap
Answers: 1
You know the right answer?
His table shows the mass and volume of four different objects.
A two-column table with 4 rows. The...
Questions in other subjects:



History, 15.01.2021 19:20

Mathematics, 15.01.2021 19:20



Mathematics, 15.01.2021 19:20


English, 15.01.2021 19:20

Mathematics, 15.01.2021 19:20