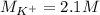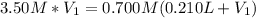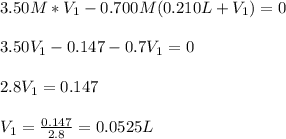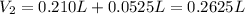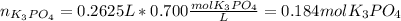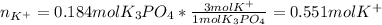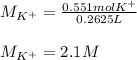Chemistry, 23.12.2020 04:30 neekobecky599
(b) An unknown volume of 3.50 M potassium phosphate, K, PO, solution is added to 0.210 L of water to form a 0.700 M K3PO, solution. Calculate the molarity of potassium ions, K in the solution.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1


Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 20:10, maddie1776
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
(b) An unknown volume of 3.50 M potassium phosphate, K, PO, solution is added to 0.210 L of water
t...
Questions in other subjects:

History, 29.08.2019 02:30

History, 29.08.2019 02:30





History, 29.08.2019 02:30

Mathematics, 29.08.2019 02:30

Biology, 29.08.2019 02:30

English, 29.08.2019 02:30