QUESTION TWO
(a) Derive Nernst equation for measuring the EMF of the cell
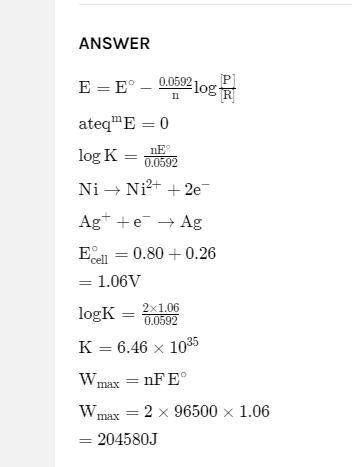
(b) Calculate the em...

QUESTION TWO
(a) Derive Nernst equation for measuring the EMF of the cell
(b) Calculate the emf of the cell
[3]
Zn Zn? (0.001M)|| Agt (0.1M)| Ag
The standard potential of Ag/Ag half-cell is +0.80 V and Zn/Zn. is -0.76 V
(c) Name different types of standard electrodes used for the determination of pH
of a solution by EMF measurement Discuss the working of Glass Electrode
for the determination of pH of a solution
(d) 0.1978 g of copper is deposited by a current of 0.2 ampere in 50 minutes.
What is the electrochemical equivalent of copper?
[3]

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
Questions in other subjects:

History, 30.09.2019 18:10



English, 30.09.2019 18:10


Chemistry, 30.09.2019 18:10