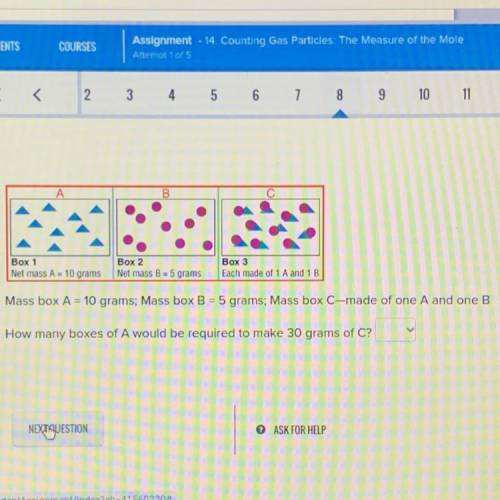
A
B
C
Box 1
Net mass A = 10 grams
Box 2
Net mass B = 5 grams
Bo...

Chemistry, 23.12.2020 01:10 Benysod402
A
B
C
Box 1
Net mass A = 10 grams
Box 2
Net mass B = 5 grams
Box 3
Each made of 1A and 1 B
Mass box A = 10 grams: Mass box B = 5 grams: Mass box C-made of one A and one B
How many boxes of A would be required to make 30 grams of C?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, elizabethprasad2
How many grams of n2h4 will be consumed by 23 g of n2o4
Answers: 1

Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Questions in other subjects:

Biology, 19.06.2020 00:57





Mathematics, 19.06.2020 00:57

Mathematics, 19.06.2020 00:57





