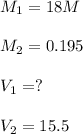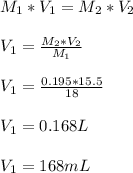What volume of 18.0 M sulfuric acid must be used to prepare 15.5 L of 0.195
M H2SO4?
none of...

Chemistry, 22.12.2020 07:00 savyblue1724707
What volume of 18.0 M sulfuric acid must be used to prepare 15.5 L of 0.195
M H2SO4?
none of these
0.336 L
168 mL
O 92.3 mL
226 mL

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:40, ellemarshall13
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2


Chemistry, 23.06.2019 14:00, jasminebaeeecx
If the molar mass of the compound is 96.69 g/mol, what is the molecular formula of the compound?
Answers: 1

Chemistry, 23.06.2019 18:30, choyontareq
You form water vapor by mixing oxygen and hydrogen at 730°c in a 5.4-liter container. this is the equation for the reaction: o2(g) + 2h2(g) → 2h2o(g). the partial pressure of oxygen before the reaction is 122.3 kilopascals, and there is excess hydrogen. how many moles of water are formed?
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 19.07.2019 10:00








Mathematics, 19.07.2019 10:00

 (Option B)
(Option B)